For the home plating specialistI cannot believe this thread is still going. Nice job, Brewsky. Plating chrome parts that are ugly is one of my favorite uses for this system. You have to buff the zinc every so often, but it sure beats ugly pitted steel and is just about an identical color to the origina chrome when buffed.
I use this process to improve some pieces as well. The rocker shafts on vintage Triumph twins stick out thru the rocker boxes and are almost always rusty. I masked off and plated the ends to avoid this on a bike I'm restoring. I suggest we all keep tricks like this in mind as we work on our bikes. regards, Rob Cold, freezing rain and sleet... good day to play in the basement with plating.
Zinc victims thrown into the vinegar yesterday as a sacrafice to the plating gods were just tiny crumbs at the bottom of the vinegar this morning. So, decided to experiment a bit. Took two old black oxide bolts and bead blasted them down to base metal. With the first one, just out of curiosity, I tried to plate it with just zinc ion rich vinegar. The absence of the conducting salts was very apparent. Voltage got to 1.5VDC and current was a measily 0.09amp. Did leave the bolt in the bath to see the outcome. It did get plated, but very lightly given that the throwing power of the bath was very poor. Added 8 oz of Epsom salts and plated the same bolt again. So this is my normal bath, but without any brightener. Bolt came out with a light gray smut that easily brushed off to reveal a very bright and evenly deposited zinc surface. Examination under a 10X loupe showed compete coverage on the thread flanks and roots. Very nice results. Added 2 grams of Knox gelatin to the bath. Gelatin was recommended by a rider on the Triumphrat site and is what he uses. I was looking for something to replace the sugar because it makes the bath a sticky PITA and makes getting good contact difficult if your set up has sat for a day or two and it now sugar coated. Gelatin was also mentioned in a techincal paper I read on plating. The gelatin did not seem to like dissolving in vinegar, but eventually did. Also makes the bath foamy as gas bubbles are given off. Plated the other bolt for the same 5 minute duration. It came out of the bath with no smut. Finish was a darker silver, more like a pewter color. I wire brushed it, but that really did nothing but maybe buff it up a bit. Conclusion for me was, I don't think I'm going to us sugar any longer. The zinc finish is just about if not identical to the bath without any brightener. I don't like the color of the zinc plate with the gelatin, so my wife benefits by my donation of the remaining packets to her cooking supplies. Here is a link to a photo showing the two bolts, with the gelatin brightener bath version being the smaller bolt on the right.  I have another member of that same forum sending a small jar of Caswell's zinc plating brightener to me to try. I think it will remove the smut, meaning no finishing brushing required. That will make plating large quantities of small parts much easier, at least on my wire brushed fingers. I'll let you know how that brightener works out. regards, Rob I just got a PM from a member here who was having problems with his plating. He was getting a dull dark gray plate. I replied to his PM, but feel that nobody on the forum benefits from exchaning PMs. I realize this thread is long and it not really a how-to, but the how-to part is buried in there amongst the exchanges. So, here is his PM to me and my reply to him in case it can help the next guy as well as perhaps fill in some of the gaps. I also realize I did not address a few points he raised in my reply to him so will do that here. Hopefully, he's following this thread and will see the additions to my reply.
Hi Rob, Read your posts on zinc plating (among others), and thought I would give it a try. So far, not having good results. I am getting a dull gray plating, not at all shiny as shown in your post. When viewed under a microscope, the surface looks speckled with zinc! I am using the 1 qt. white vinegar + .25 lb. epsom salt + .25 lb. sugar formula. "Zinc Shield" roofing strips @ 99.1 % zinc, 2.5 " wide. This is all set up in a plastic container of about 1 qt. capacity 7" high x 6" dia. There are 4 zinc strips in the jug that pretty much enclosed the whole inside. Tried the following power supplies: plug-in wall unit 3 volts D.C. @ 300 milliamps; two "D" cells in series (3 v.); 1 "D" cell; two "C" cells in series & finally one "C" cell. The different cells all produce a gray color, but lower power level seems a bit better. Tried experimenting with plating time to no avail. Used from 1 minute to 30 minutes. I am getting what I think is rapid wear of the zinc strips. In about 1 hour of total running the strips went from about .007" to .002". When there is no current present I notice that there is still bubbling coming off of the zinc strips. Where I am right now is that I am thinking that my container is too small & am getting too much zinc transfer even at low power levels. Am thinking of making up a new batch of electrolyte and using a bigger container. By the way, started out trying to plate a small part (petcock retainer plate & 10 penny nail). Any reccommendations on what I might try next would be greatly appreciated. Regards, First, although it is long, I would suggest you go back and read the entire thread. I'm 99% sure that the answers to all your questions and more are in that thread. I know it is long, but a worthwhile read. I was just doing some plating today by coincidence. I've used this process quite a bit since first discovering the thread a few years ago and have learned a bit more in the process of doing it. First, you must monitor your amps and your voltage. You cannot just plug in any power supply and get consistent results. All of my experience and from what I've read on this topic, for what we are doing, 1.5 VDC is a high a voltage as you want to use. Once you start to exceed that amount, the plating gets dark and mottled. I've had it happen to me when I've had anomalies cause me to lose control of the voltage. You need to control the amps because your power supply will have an amp limit. If you exceed the limit, you'll smoke the power supply. When I plate, I set up two DMMs one reading DC volts and the other reading amps. I'm pretty sure I posted a circuit diagram in that thread. I have a large Ohmite rheostat in series with the power supply to control the power. I control by either hitting the 1.5 VDC limit or the 1 amp limit of my power supply, which ever comes first. The size of the piece being plated determines the volt-amp relationship. For small pieces you will hit the volt limit first. For larger pieces you will hit the amp limit. If your power supply is bigger than mine, you can go to higher amps. More amps normally means more throwing power. If you do it right, the part should have a flat light gray finish. Brush it with a brass wire hand brush and it will remove that flat gray smut and leave a nice silver zinc finish. I just stopped using sugar as a brightener. I'm sick of the sticky mess it makes everytime you drip bath from a part. All my meters, my rheostat, my power supply were candy coated. I cleaned them off the other day and tried plating without any sugar in the bath. The part comes out with the same flat, light gray finish and I just brush it shiny. I have a bottle of Caswell brightener coming from another rider on the Triumph forum. It may preclude the need to brush the part. That will be handy when doing large quantities of small parts because I hate the brushing part and my hands take a beating from the brush. I use a 1 amp, 12 volt power supply. It was for an old modem I had. Been using it for 2 years now. I started with a smaller one, but kept pushing the amps to get more throwing power and smoked it. Hope this helps. Here's a copy of the circuit diagram. 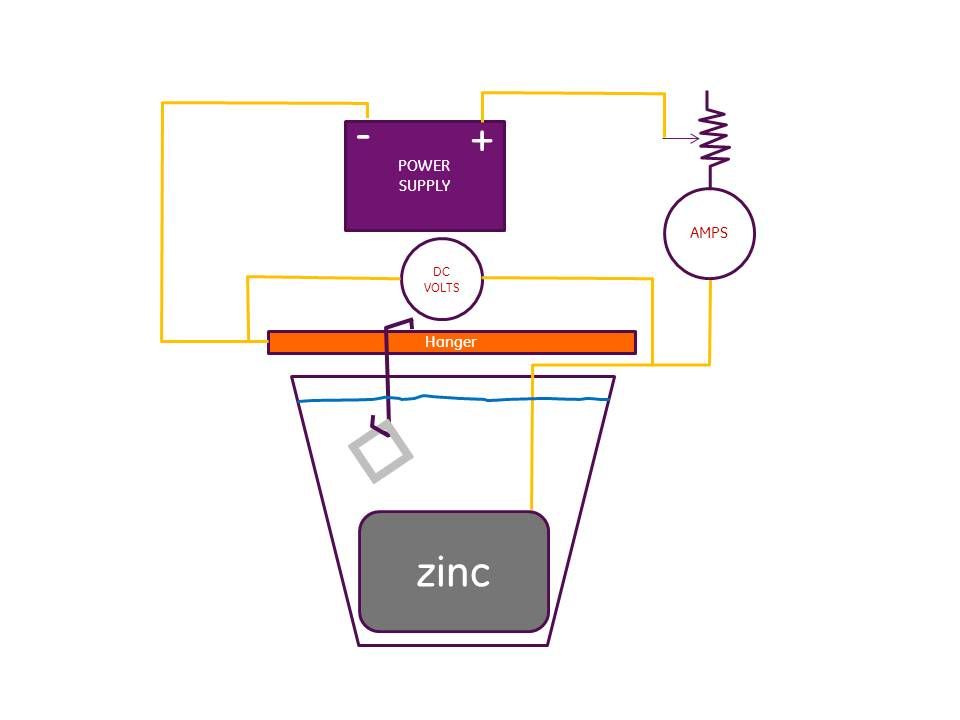 Oh, and one last thing. I'm convinced that the best way to set up your tank is with a cylinder of zinc sheeting. My tank is bascially a plastic bucket with a bottomless zinc bucket inside it made from zinc sheet. Plating is line of sight so this gives you 360 degrees of throwing ability. It makes all the parts come out uniformly plated. ADDED INFO: The bath when first made has no zinc ion. So, the bath will eat away your zinc in the tank rather quickly at first. So, I drop some pieces of zinc into the bath the night before I pour the bath into the tank. And keep in mind that as long at the bath is in the tank, it will eat the zinc, maybe at a slower rate as it loads up with zinc ions, but still eats it. So, when you are done, pour the bath back into the vinegar jug. Your zinc will last longer that way. My first zinc was gone quickly. 2 years later, I'm still using my second zinc set-up. I've never tried to do this process without controlling the power with a rheostat. First one I used was a small pot I scavenged from an old stereo. It would get too hot to touch, and the adjustment was very coarse. But it did the job until I got a bigger rheostat. So, I'm not sure what having 3 VDC in battery form will do, but suspect it will deliver 3 VDC and whatever amps the system demands. That could give you a dark gray result. As you read thru the entire thread, take notes on tips and process details as they are shared. It's your best way to get as much value from our experiementation and experience as possible. regards, Rob Hi Rob,
Your last 2 posts are clear and informative as usual. Just a couple of observations: I've never experienced the sugar stickiness that you speak of and wonder if your recipe was flawed, not that it matters much since you are now going 'sugar-free'. The second relates to the pm. His bucket capacity (1 qt) suggests the part being plated is very close to the zinc strips and that proximity might be a factor in his poor plating quality. The law of inverse squares is operative here--half the distance to the zinc results in a 4 fold rate of transfer. A larger bucket diameter might help (mine is about 9"). Wilf Thanks Wilf. I have to admit that I've been remiss in researching and understanding the math involved in plating. I know that there are calcs that can be done that involve surface area to determine the correct voltage and current. But I have had such good luck with my process to date that I've had no interest in confusing myself with more facts. That's rare for me, but plating is a means to an end, not a passion in and of itself for me.
Interesting what you are saying about proximity to the zinc. I've not experienced that in any way. When I was doing my CL77 parts, I went so far as to plate the inside of some tubular pieces and large nuts by making a zinc that would fit into the ID and the part rest on a rubber insulator. I used a small butter tub as a tank. Maybe held 1 pint. Every part came out fine. The other day I was doing the oil feed pipe for my '66 Triumph T120R and made the tank from a piece of plastic rain gutter and lined it with zinc. I put small rubber stoppers in the two banjo fittings to prevent the part from shorting on the zinc. This put the part extremely close to the zinc. Yet I observed no indication of extra heavy deposition. Any thoughts? I'm hesitant to ask because I'm happy with the way everything is working. But you've never steered me wrong and I might be doing something without realizing it or my set up may be perfect purely by chance. I know that the cylindrical zinc seems to do the best job for general plating. My tank holds 2 quarts. regards, Rob
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||


